The Dallas area has been hit especially hard by COVID-19. As of November 9, 2020, a total of 112,000 cases and over 1300 deaths have occurred. The pandemic is refusing to go down without a fight. But then again, neither are we humans.
With viruses as prevalent as COVID-19 becoming an unwanted part of everyday life, the smallest residence to the largest of corporations needs to think about a strong disinfection plan. Eating surfaces, countertops, tables, you name it, all need to be properly cleaned to prevent infection.
Medical facilities are certainly at the epicenter for areas that need constant cleaning and disinfecting, along with sterilization of all their medical instruments.
But there are other areas and facilities that are thought of less often such as disinfecting museums, veterinary practices and retail stores. People come and go all day long into their residences, workplaces, and businesses. But how often are those areas cleaned? This is where high-level disinfection comes into play.
What is High-Level Disinfection?
The FDA defines “disinfection” as “The destruction of pathogenic and other kinds of microorganisms by physical or chemical means.”
“Disinfection is a less lethal process than sterilization since it destroys most recognized pathogenic microorganisms, but not necessarily all microbial forms, such as bacterial spores. Disinfection processes do not ensure the margin of safety associated with sterilization processes (AAMI, 1995).”
The FDA is the only authority that regulates high-level disinfectants used for disinfection.
High-Level – This level of disinfection is commonly used in hospital settings when people like doctors, nurses, or emergency personnel in an ambulance have to deal with open wounds or similar. HLD helps to ensure that pathogens and harmful bacteria in those areas are kept under control.
Spaulding Classification: High-Level Disinfection of Medical Devices
The main steps for disinfecting medical instruments were outlined In a 1939 paper titled “A Rational Approach to Disinfection and Sterilization,” by Temple University student Earle H. Spaulding. These very logical steps for disinfection are still used today with some refinements.
The Spaulding classification divides medical devices into three categories: non-critical, semi-critical, and critical, based on their potential to cause healthcare-associated infections (HAIs).
Devices are classified as non-critical if they are used on intact skin and pose the lowest risk of causing HAIs.
Semi-critical devices carry a moderate risk for infection as they come into contact with mucous membranes or non-intact skin.
Critical devices have the highest risk of causing infections and come into contact with sterile body sites such as the bloodstream, heart, and brain. It’s worth noting that a device’s classification can change based on its specific use. For example, a transabdominal ultrasound probe may be considered non-critical when used on intact skin but would be classified as semi-critical when used on a visible skin lesion.
Non-Critical
Non-critical devices used on intact skin:
- Stethoscopes
- Blood pressure cuffs
- Transabdominal ultrasound probes
Cleaning, low-level disinfection, or intermediate-level disinfection can use household bleach, alcohols, Iodophor Phenolics, and quaternary ammonium-based disinfectants.
Noncritical items include things that come into contact with intact skin, such as clothing, floors, high-touch surfaces, furniture, baths, bed pans, weighing scales, brushes, beddings, crockery, earphones, mobiles, and trolleys.
Although the risk of infection transmission from these items is low, they can still indirectly contribute to the spread of infection. For instance, patient belongings, like clothing and cell phones, can harbor dangerous bacteria such as MRSA and VRE, which can be transferred to other patients by healthcare workers’ hands.
While these low-level disinfection items don’t require sterilization, regular cleaning and disinfection with low-level disinfectants (LLD) can help reduce the spread of infectious organisms.
Semi-Critical
Semi-critical devices are items that come in contact with mucous membranes or non-intact skin.
- Transvaginal and transrectal ultrasound probes
- Transesophageal echocardiogram probes
Chemicals such as glutaraldehyde, ortho-pthaldehyde, peracetic acid, hydrogen peroxide, and hypochlorite may be sufficient for high-level disinfection and cleaning.
Semi-critical items should be rinsed with sterile water or alcohol. Utilizing forced air drying after the rinsing process significantly reduces the contamination rate while cleaning has been shown to reduce the transmission of infection in HIV-contaminated instruments.
Items are found to be free of germs when soaked in 2% glutaraldehyde for 20 minutes after cleaning. OPA, glutaraldehyde, and an automated process using PAA are the three high-level disinfectants commonly used to reprocess endoscopes.
Critical
Critical Devices that come in contact with sterile body sites:
- Implanted devices
- Surgical instruments (scalpels, forceps, etc.)
Critical items are cleaned and sterilized using peracetic acid, hydrogen peroxide, steam, ethylene oxide, hydrogen peroxide, and gas plasma.
Devices that come into contact with the sterile parts of the body are considered critical items because they pose the highest risk of transmitting infection. Therefore, sterilization is the preferred method for reprocessing these items, especially those that can withstand heat.
The FDA has approved ethylene oxide (EtO), plasma sterilization, and liquid sterilization with glutaraldehyde or PAA for heat-sensitive items. All properly sterilized items should be handled with care to avoid environmental contamination.
4 Examples of High-Level Disinfection
High-level disinfection in medical and residential areas presents various challenges. Ensuring that all surfaces and equipment are thoroughly disinfected to prevent the spread of infections is crucial yet complex.
Different areas, such as patient rooms, surgical areas, and shared spaces, require specific approaches to high-level disinfection. Effectively managing these challenges is essential for maintaining a safe and hygienic environment for patients, residents, and healthcare workers.
Manual vs Automated High-Level Disinfection

High-level disinfection can be accomplished through either a manual or automated process. In a manual high-level disinfection process, a device is usually immersed for a specified time at a specific temperature and concentration.
However, according to ANSI/AAMI ST91:2021, using a manual process is not recommended as it can lead to greater variation and inconsistency in the disinfection process. Automated processes offer more efficient, consistent, and measurable results while reducing staff exposure.
Cleaning and Disinfection of Blood Spills

Using ILD or high-level disinfectants with tuberculocidal activity is crucial when cleaning and decontaminating hospital surfaces contaminated by spilled blood.
- For small blood spills (<10 mL), use a 1:100 dilution of sodium hypochlorite solution.
- For larger spills >10 mL, the solution should be a 1:10 dilution for the initial application.
Remove any organic material with absorbent material and then proceed to thoroughly disinfect using chemical disinfectants such as a solution of sodium hypochlorite diluted at a ratio of 1:100.
High-Level Disinfection of Medical Instruments
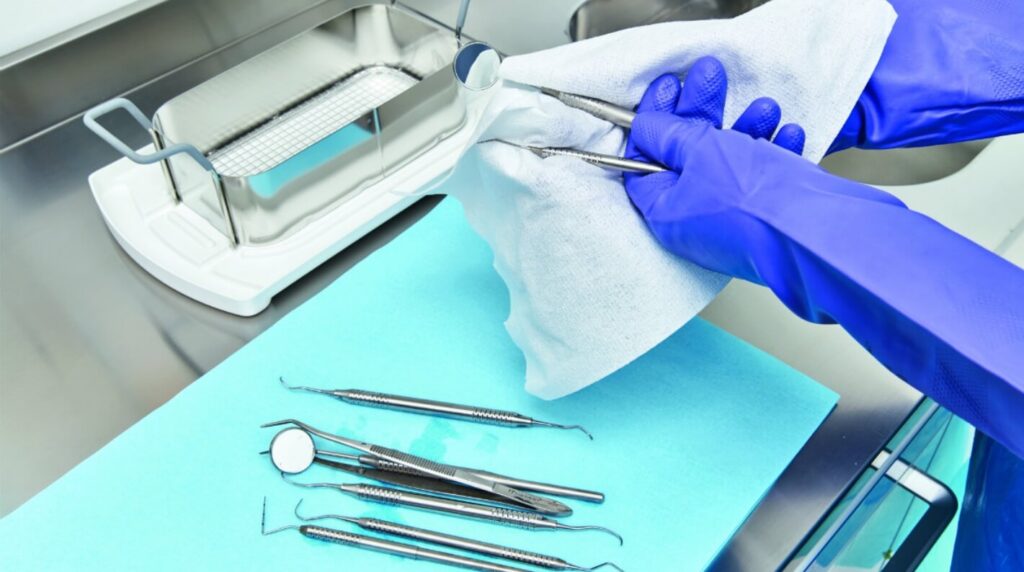
Consider the physical characteristics and materials of medical equipment when cleaning and disinfecting it. Thorough cleaning is essential for removing the majority of microbes from the equipment before using high-level disinfectants.
To ensure consistency and a high standard of care, staff should receive proper education and training on cleaning procedures, the physical and chemical nature of instruments, and the properties of chemical disinfectants. During the cleaning process, all staff should use personal protective equipment (PPE) to ensure their safety.
Removing dry organic materials from instruments can be challenging. To address this, it’s best to avoid drying by immersing the equipment in a detergent or disinfectant solution before cleaning. The soaked matter can then be manually scrubbed, rubbed with a brush, or cleaned with an automated scrubber, followed by a thorough wash with water under pressure.
While a short soak can be beneficial, avoiding prolonged or overnight soaking of the devices is important.
Maintaining the detergent’s proper concentration and exposure time or high-level disinfectants is crucial for effective cleaning. If the concentration is too low it may not effectively remove organic materials or microorganisms.
Additionally, it’s important to ensure that the disinfectant’s pH aligns with the manufacturer’s instructions of the article. Delicate articles should be processed in a neutral pH solution.
Enzymatic cleaners with a neutral pH can accelerate the cleaning process without damaging the articles. For example, a neutral pH detergent with enzymatic action is preferred when cleaning a flexible endoscope.
A new non-enzymatic product based on hydrogen peroxide, cleared by the US Food and Drug Administration (FDA), is very effective as a cleaning agent.
Disinfection of Endoscopes
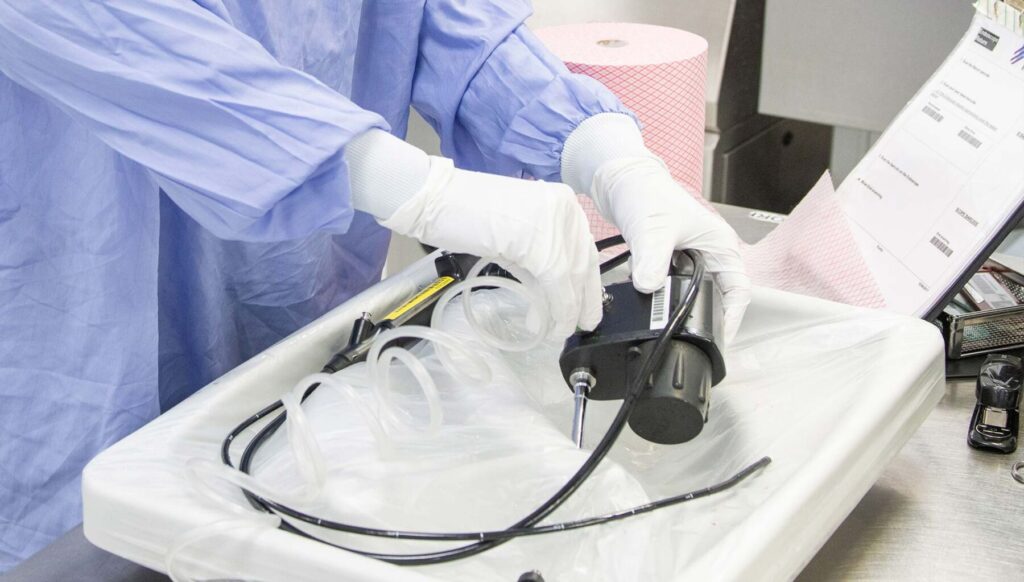
Physicians commonly use endoscopes to diagnose and treat a wide range of internal medical conditions. Although they are highly valuable in modern medicine and have a reported infection rate of less than 1 in a million procedures, endoscopes contaminated with bacterial spores have been associated with 97 more healthcare-related outbreaks than any other medical device.
The Centers for Disease Control (CDC) recommends that all heat-sensitive endoscopes, gastrointestinal endoscopes, bronchoscopes, and nasopharyngoscopes must be thoroughly cleaned with high-level disinfectants after every use to prevent the spread of healthcare-associated infections.
High-level disinfection is expected to eliminate all microorganisms, although a few spores may survive in cases with a high number of bacterial spores.
What Chemicals Are Involved with High-Level Disinfection?
High-level disinfectants are normally only found in hospital settings. These chemicals are very useful and effective when used the correct way. They can also be used in all sorts of businesses and even residential settings. When used as a disinfectant, only trained personnel should deal with these chemicals as they need to be mixed properly.
Some of the ingredients used are:
- Hydrogen Peroxide – An extremely useful disinfectant that’s normally used on material surfaces.
- Hypochlorous Acid – Literally chlorine mixed with water. It makes for an excellent cleaner.
- Glutaraldehyde – is used for many things, from treating warts to sterilizing surgical instruments.
High-Level Disinfection in Residential Areas
One of the most common areas for High-Level Disinfection in the home would involve carpet cleaning. The Institution on Inspection, Cleaning and Restoration Certification (IICRC) provides industry standards and recommendations for things like cleaning carpets, for instance. The IICRC suggests that carpets in the home be cleaned every eight to twelve months, but with shorter frequency when dealing with an epidemic, for instance, maybe every six months.
HLD is also essential when cleaning abandoned homes before renovation. Anyone who’s ever watched the television show ‘Hoarders’ has probably seen the specialized teams of people wearing full-hazard gear going in to get the job done.
Carpets attract so much dirt and debris that a thorough cleaning is essential. Microbes, bacterial spores, bugs, and more can hide in carpets for a very long time. If you have children and/or pets, you’ll want to make sure those areas stay safe. When you hire a professional cleaning service like the team at Dallas Janitorial Services, you can be assured that they will use the proper equipment to clean and disinfect your carpet and regular floors.
Fabric curtains are another thing that is infrequently cleaned, but they do collect a huge amount of dust, mites, and other particles every day. Professional cleaning services can normally clean curtains with steam and disinfectant without having to remove them. The same can be said for cloth-covered furniture and cushions. And don’t forget those window blinds!

Disinfecting Commercial Buildings
HLD cleaning is absolutely essential for any business these days. It’s especially handy for high-traffic zones such as public restrooms. Frequent areas like walls, light switches, and floors need to be disinfected. Public sinks can be a hotspot for bacterial spores and mold. Company cafeterias, meeting rooms, and even the office printer should be cleaned also. It’s always better to be safe than sorry when it comes to keeping your common business areas and employees protected from harmful pathogens.
In medical facilities, there are no areas that should be left out when performing high-level disinfection. Places like hospitals are so busy, it’s a real challenge to keep up with things like disinfecting areas 24/7.
Businesses like daycare centers and retirement homes need to stay especially vigilant when it comes to high-level disinfection. Viruses like COVID-19 can run like wildfire through facilities like those. This is the main reason why industrial cleaning services like Dallas Janitorial Services exist.

Get in Touch with Dallas Janitorial Services
With our wide range of disinfection services, we at Dallas Janitorial Services know we can help your residential or business be as clean as it can possibly become. Our highly trained team of cleaning experts and help you devise a plan that works for your services.
From carpet cleaning to wall disinfection to fogging, we’ll prove how effective our techniques can be. We serve the entire Dallas/Ft. Worth the area, no matter how big or small. Contact us for more information at any time.
FAQs About High-Level Disinfection
Here are some frequently asked questions about high-level disinfection.
Which is most often used as a high-level disinfectant?
High-level disinfectant products usually consist of a combination of chemical disinfectants such as bleach and hydrogen peroxide or a blend of peracetic acid and hydrogen peroxide.
According to the CDC, peracetic acid and hydrogen peroxide are some of the most common active ingredients in high-level disinfectants.
What are the three levels of disinfection?
There are three levels of disinfection: high, intermediate, and low-level disinfection. The high-level disinfection (HLD) process kills all vegetative microorganisms, mycobacteria, lipid and nonlipid viruses, fungal spores, and some bacterial spores.
What requires high-level disinfection?
Both critical and semi-critical items require a high level of disinfection. Critical items enter sterile body sites or the vascular system and require sterilization. Semi-critical medical devices contact mucous membranes or non-intact skin and should undergo high-level disinfection if sterilization is not possible.
What is the difference between sterilization and high-level disinfection?
High-level disinfection and sterilization are both decontamination processes. Disinfection eliminates or reduces harmful microorganisms from objects and surfaces, while sterilization kills all microorganisms.


